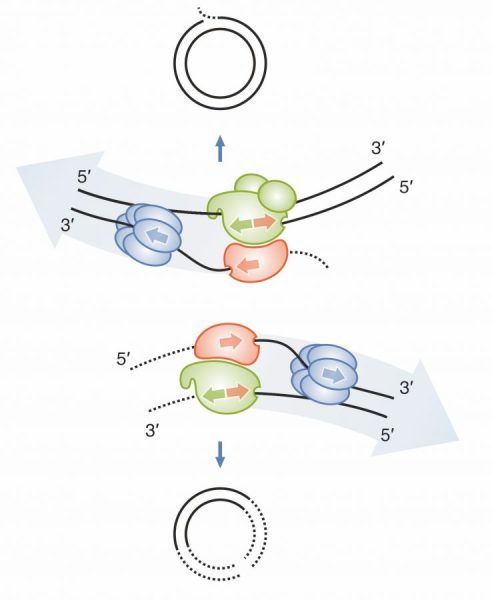
If you want to know what happens when the circular mitochondrial genome breaks and suddenly has two ends (aka linear), take a look at our new paper in Nature Communications. Would you ever believe that exonucleases famous for their fine sculpting abilities could be so brutal?
Archive for the ‘genome’ Category
When the wheel breaks
May 1, 2018Link to MitoWheel
May 12, 2016If you have a website dealing with human mitochondrial DNA and you would like to show specific positions graphically, you can now make a direct link to MitoWheel. Simply send your query through the HTML address line appending a question mark and the query text. Something like this:
http://mitowheel.org/mitowheel.html?4277C
or
http://mitowheel.org/mitowheel.html?tRNALeu
Small addition: using the left and right arrow keys on your keyboard, you can now walk through the sequence nucleotide by nucleotide. If you want to make bigger jumps, type into the search field ‘+42’ or ‘-17’ and press ENTER repeatedly.
MitoWheel 2.0
April 5, 2016MitoWheel was updated on April 5th, 2016.
![]() Well, it is actually more than just an update. MitoWheel has been re-made from scratch and is now fully based on Java. This means that you will be able to use it on mobile devices or some smart TVs. The actual version contains all features that I myself regularly use. Nevertheless, some functions of the old version are not yet implemented. If you cannot do without them, you will find a link on the ‘About’ page to the predecessor. This you can access by clicking on the ‘+’ sign. New is the orange button in the search field that serves for launching a query or walking through multiple matches, things that otherwise can be done by pressing ENTER. On the other end of the search field, you will find the search icon, similar to the old version. New is that you can click on it to load files with lists of queries, most importantly with lists of SNPs. The files should be simple text files in FASTA format (‘>Sequence_Name’ followed by a comma-separated list of SNPs in one or more lines) or VCF-type text files that are used to represent exome sequencing data. If you would like to use file types that do not load into MitoWheel 2.0, email me a sample, and I will do my best to extend its file reading capabilities.
Well, it is actually more than just an update. MitoWheel has been re-made from scratch and is now fully based on Java. This means that you will be able to use it on mobile devices or some smart TVs. The actual version contains all features that I myself regularly use. Nevertheless, some functions of the old version are not yet implemented. If you cannot do without them, you will find a link on the ‘About’ page to the predecessor. This you can access by clicking on the ‘+’ sign. New is the orange button in the search field that serves for launching a query or walking through multiple matches, things that otherwise can be done by pressing ENTER. On the other end of the search field, you will find the search icon, similar to the old version. New is that you can click on it to load files with lists of queries, most importantly with lists of SNPs. The files should be simple text files in FASTA format (‘>Sequence_Name’ followed by a comma-separated list of SNPs in one or more lines) or VCF-type text files that are used to represent exome sequencing data. If you would like to use file types that do not load into MitoWheel 2.0, email me a sample, and I will do my best to extend its file reading capabilities.
Short summary of features:
- Navigate by clicking on the point of interest either on the sequence bar or on the Wheel
- If you are bored, you can just flick the sequence bar or the Wheel. You have won if it stopped in the anticodon of a tRNA (chances are 66 to 16569)
- Search for nucleotide sequence motifs, now including ambivalent positions. You can either use usual IUPAC nucleotide codes (‘R’ for ‘A’ or ‘G’, ‘Y’ for ‘C’ or ‘T’, etc.) or indicate the alternative nucleotides in square brackets (‘AC[GC][AGCT]CC[AT]GC’)
- Search for terms like ‘protein’, ‘MT-TS2’, ‘complex IV’
- If you want to search for multiple terms, separate them by comma
- Load files by clicking on the search icon
- The purple frequency bars, located below or above each nucleotide, are now calculated based on 30,181 human database sequences. Exact numbers are displayed if you search for specific alleles (‘5846C’ or ‘m.12367A>G’)
Note on privacy: Once loaded into your browser, MitoWheel 2.0 functions entirely offline. No data are sent through the net. In case some future functions will require a connection to the server, this will be explicitly indicated.
I hope you will have fun with MitoWheel 2.0. If you miss some features, either from the old version or something that just crossed your mind, do not hesitate to email me. MitoWheel is probably not free of errors. If you notice one, please let me know. Just as in the last eight years, keep the Wheel rolling!
MitoWheel: The Manual
April 6, 2008The MitoWheel was updated on April 6th, 2008.
There were small corrections made to the program. However, the most relevant change happened to the web site. I deleted the words ‘Under construction’ in the section ‘How to use’, and I wrote quite a lot of other words instead. Most of the instructions and tricks have been already mentioned here in the blog, but now you can find a more systematic guide right there where the Wheel is. To make the manual more enjoyable, I also included three short videos showing the Wheel in action.
As an appetizer, here you can see the first video about navigating the Wheel. For better quality, check out the original.
A Little Make-Up
March 30, 2008The MitoWheel was updated on March 30th, 2008. This time new:
- After a few years of hesitation, I finally switched the orientations of the two replication origins. If you prefer the previous version, or you find some evidence that the previous version was more correct, please let me know.
- Now you can really spin the Wheel directly! Just click on it and drag it around.
Sequence Bar with Allele Frequencies
March 23, 2008The MitoWheel was updated on 23th March, 2008. This time new:
The sequence bar now contains information not only about the function of specific nucleotides, but also about allele frequencies at polymorphic positions. Each little gray bar above or below a nucleotide letter represents the number of individuals who carry a difference (also known as SNP) at the given position as compared to the revised Cambridge reference sequence. To be able to show both very rare and very frequent polymorphisms, the bars have a logarithmic scale. I hope you will find this new feature useful. This can help you to design reliable PCR primers for the human mitochondrial genome. After all, you don’t want your primer’s 3′-end sitting right on a very frequent polymorphism (risking that under certain conditions you will not be able to amplify a PCR fragment from a subset of individuals). The allele frequency bars are also good starting points if you want to explore the diversity of the human population by using the “+” and “-” operators in the search box (as described here).
I hope you will find this new feature useful. This can help you to design reliable PCR primers for the human mitochondrial genome. After all, you don’t want your primer’s 3′-end sitting right on a very frequent polymorphism (risking that under certain conditions you will not be able to amplify a PCR fragment from a subset of individuals). The allele frequency bars are also good starting points if you want to explore the diversity of the human population by using the “+” and “-” operators in the search box (as described here).
MitoWheel.org
March 15, 2008If you tried to use the MitoWheel in the last two days, you might have noticed that the site was down. I am very sorry for the inconvenience. The hosting server had serious technical problems. Well, those problems are still not solved, but in the meantime I changed provider. And if I already had to change, I used the opportunity to create a new domain. Let me introduce you the new home of MitoWheel: MitoWheel.org.
The old address is supposed to work again very soon. However, after a few weeks of transition I will discontinue with it, and MitoWheel.org will be the only place to find the MitoWheel. So don’t forget to change your bookmarks. If you notice any technical issues, please report it to me.
Have fun spinning the Wheel!
MitoWheel 1.2: Humankind in the Wheel
March 9, 2008… or at least those individuals whose complete mitochondrial DNA sequence can be found in GenBank. MitoWheel was updated on March 8, 2008. New features:
- MitoWheel incorporates now data on fully sequenced human mitochondrial genomes that have been deposited in the GenBank nucleotide database by different research groups. This means at the moment 2982 complete human mitochondrial DNA sequences. You can type the GenBank accession number into the search field and you will get the list of mutations (positions that are different from the reference sequence). Of course, you can see multiple sequences at the same time (“AY665667, DQ862537”). In this case, the sequences will be distinguished by color. Well, you might ask, who will really search for DQ862537? Probably, you are right… But don’t stop reading!
- MitoWheel 1.2 is able to show a number of human mitochondrial sequences as a group. In the group view, mut
 ations that occur in any of the sequences in the group are divided into four classes: (a) Mutations that are specific for the group, i. e. they cannot be found in any other known human mtDNA sequence, only in the analysed group. (b) Mutations that are present in all sequences belonging to the group (“ubiquitous“), but also occur outside of the group. (c) Mutations that are missing from some of the sequences, so they define a subgroup within the group. (d) At last, the status of certain sites is shown, although they are identical to the revised Cambridge reference sequence (rCRS). It is necessary, because the reference sequence itself carries some polymorphisms that actually represent a minority within the human population. At such nucleotide positions, where sometimes 98% of the human mtDNA sequences differ from the reference sequence, the lack of the “mutation” is more informative than its presence. For the same reason, rCRS polymorphisms are faded out if they fall into the “ubiquitous” group. Color intensity gives additional information about “specific” and “subgroup” mutations too: those that are carried by many individuals in the group are shown in bright color, while rare ones are faded out. You can also find exact numbers about these features in the info window: next to the name of the mutation, frequency data are shown. The first pair of numbers says how many sequences carry the mutation within the group, and the second pair of number describes how frequent the mutation is in the entire human population.
ations that occur in any of the sequences in the group are divided into four classes: (a) Mutations that are specific for the group, i. e. they cannot be found in any other known human mtDNA sequence, only in the analysed group. (b) Mutations that are present in all sequences belonging to the group (“ubiquitous“), but also occur outside of the group. (c) Mutations that are missing from some of the sequences, so they define a subgroup within the group. (d) At last, the status of certain sites is shown, although they are identical to the revised Cambridge reference sequence (rCRS). It is necessary, because the reference sequence itself carries some polymorphisms that actually represent a minority within the human population. At such nucleotide positions, where sometimes 98% of the human mtDNA sequences differ from the reference sequence, the lack of the “mutation” is more informative than its presence. For the same reason, rCRS polymorphisms are faded out if they fall into the “ubiquitous” group. Color intensity gives additional information about “specific” and “subgroup” mutations too: those that are carried by many individuals in the group are shown in bright color, while rare ones are faded out. You can also find exact numbers about these features in the info window: next to the name of the mutation, frequency data are shown. The first pair of numbers says how many sequences carry the mutation within the group, and the second pair of number describes how frequent the mutation is in the entire human population. - How to create groups of human sequences? By mutations! Select all sequences that carry a certain mutation, or select exactly those that lack it. You just simply have to type a “+” or a “-” in front
 of the name of the mutation. For example, “+4917G, +1888A, -12633A, -930A” means “include sequences that carry 4917G and 1888A, but lack both 12633A and 930A”. This will give a nice little group of 24 sequences with many characteristic mutations (“ubiquitous”), and a dark blue “subgroup” mutation, 11812G, that is missing only from one of the sequences (“23/24” in the info window). So why don’t you check out that subgroup by typing “+4917G, +1888A, -12633A, -930A, +11812G”? Are you aware that you are doing phylogenetics right now? You landed in a subgroup of the human T2 subclade in haplogroup T.
of the name of the mutation. For example, “+4917G, +1888A, -12633A, -930A” means “include sequences that carry 4917G and 1888A, but lack both 12633A and 930A”. This will give a nice little group of 24 sequences with many characteristic mutations (“ubiquitous”), and a dark blue “subgroup” mutation, 11812G, that is missing only from one of the sequences (“23/24” in the info window). So why don’t you check out that subgroup by typing “+4917G, +1888A, -12633A, -930A, +11812G”? Are you aware that you are doing phylogenetics right now? You landed in a subgroup of the human T2 subclade in haplogroup T.
MitoWheel 1.1 Goes Rainbow
February 10, 2008The MitoWheel was updated on February 10th, 2008. New features:
- You have already discovered for sure that the MitoWheel is able to show multiple results from any query. You get such multiple results if you type something like “tRNA” or “AGCTA”. You can then walk through
 the results by clicking the red arrow in the search field, or simply pressing ENTER over and over. With the MitoWheel 1.1, we are going one level higher: you can now see several lists of results at the same time. As you might guess from the title of this blog, the different groups of matches are distinguished by color. For example, you can try “complex I, complex III, complex IV, complex V” (as a matter of fact, “c1, c3, c4, c5” works as well). You will get all the mitochondrially encoded subunits of these protein complexes, each complex in another color. To find out what each color stands for, take a look at the lower left corner. The only rule to make multiple searches is: separate the terms with commas.
the results by clicking the red arrow in the search field, or simply pressing ENTER over and over. With the MitoWheel 1.1, we are going one level higher: you can now see several lists of results at the same time. As you might guess from the title of this blog, the different groups of matches are distinguished by color. For example, you can try “complex I, complex III, complex IV, complex V” (as a matter of fact, “c1, c3, c4, c5” works as well). You will get all the mitochondrially encoded subunits of these protein complexes, each complex in another color. To find out what each color stands for, take a look at the lower left corner. The only rule to make multiple searches is: separate the terms with commas. - If your search returns a region that is longer than 30 nucleotides, it will now be shown as an arc.
- Do you want to see, why the two strands of the mitochondrial DNA are called “heavy” and “light”? Type “CCC, GGG” and press ENTER. Cytosine has a molecular weight of 111.10 g/mol, while guanine 150.13 g/mol. The strand with more guanines is, therefore, heavier than the one with mainly cytosines. (Please note that most genes on the mitochondrial genome are physically located on the heavy strand. If it looks the other way around, it is because gene sequences are always given as the complementary strand that corresponds to the mRNA that is transcribed from the gene.)
- If you really want a lot of colors, try this: “TTTT, AAAA, CCCC, GGGG”. Then this: “I love mtDNA, TTTT, AAAA, CCCC, GGGG”, and this: “I love mtDNA, and the MitoWheel too, TTTT, AAAA, CCCC, GGGG”. (Well, you can skip the nonsense, it will be ignored anyway. But keep the extra commas to shift the color scheme.) If you make some search that turns the MitoWheel into a colorful piece of art, send me the text of the query and the title of your mito-painting. We could make a gallery…
Cut and Paste the Human Mitochondrial DNA
January 30, 2008The MitoWheel was updated on January 30, 2008. New features:
- Beside positions, regions, gene names, general terms, or sequence motifs, now you can type into the search field mutations too. What happens is not very different from that when you are searching for a specific position: the wheel moves to the corresponding position. The change is in the info window. If your position is in a protein coding or a tRNA coding region, you will get an additional info on the functional change caused by the mutation. Mutations are accepted in the following forms: 12276A, G12276A, 12276G>A, or if you are a perfectionist m.12276G>A.
- The results of your queries can be now sent to the clipboard of your operating system. If a search returns a region (i.e. more than just one nucleotide position) a small sign appears in the upper right corner of the info window. If you click on this sign, the sequence will be saved in the clipboard. You can now easily paste it into any other program.
Tipp: If you more or less found the region on the wheel that you were searching for, and want to make some fine navigation, try the following possibilities:
- Click on the left or right arrows quickly.
- Press briefly the left or right buttons on your keyboard.
- Click on a nucleotide in the sequence bar. This nucleotide will move to the center.